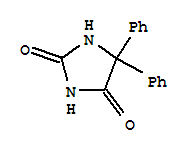
5,5-二苯基乙内酰脲 修改号码:5
模块 1. 化学品
产品名称: 5,5-Diphenylhydantoin
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
急性毒性(经口) 第4级
生殖细胞敏感性 1B类
致癌性 第2级
生殖毒性 1A类
特异性靶器官毒性 神经系统
- 单一接触 [第1级]
特异性靶器官毒性 肝脏, 神经系统, 牙龈, 淋巴腺
- 单一接触 [第1级]
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 危险
危险描述 吞咽有害。
可能导致遗传性缺陷
怀疑会致癌
可能损害生育能力或胎儿
对器官引起损害: 神经系统
可能因延长或接触对器官产生损害: 肝脏 神经系统 牙龈
淋巴腺
防范说明
5,5-二苯基乙内酰脲 修改号码:5
模块 2. 危险性概述
[预防] 使用前获取特定手册。
处理前必须阅读并理解所有安全措施。
切勿吸入。
使用本产品时切勿吃东西,喝水或吸烟。
处理后要彻底清洗双手。
使用个人所需的防护用具。
[急救措施] 食入:若感不适,呼叫解毒中心/医生。漱口。
如接触到或相关接触:求医/就诊。
[储存] 存放处须加锁。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 5,5-二苯基乙内酰脲
百分比: >99.0%(T)
CAS编码: 57-41-0
俗名: Phenytoin
分子式: C15H12N2O2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。
求医/就诊。
食入: 求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用特殊的个人防护用品(针对有毒颗粒的P3过滤式空气呼吸器)。远离溢出物/泄露
紧急措施: 处并处在上风处。
泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果可能,使用封闭系统。如果粉尘或浮质产生,使用局部排气。
5,5-二苯基乙内酰脲 修改号码:5
模块 7. 操作处置与储存
操作处置注意事项: 避免所有部位的接触!
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
存放处须加锁。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统。同时安装淋浴器和洗眼器。
个人防护用品
呼吸系统防护: 防尘面具,自携式呼吸器(SCBA),供气呼吸器等。使用通过政府标准的呼吸器。依
据当地和政府法规。
手部防护: 防渗手套。
眼睛防护: 护目镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防渗防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色类白色
气味: 无资料
pH: 无数据资料
熔点: 298°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 不溶于
[其他溶剂]
微溶于: 酒精, 丙酮
不溶于: 醚, 苯, 氯仿
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx)
模块 11. 毒理学信息
急性毒性: ivn-rat LD50:101 mg/kg
orl-rat LD50:1635 mg/kg
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: dna-esc 50 umol/L
dni-hmn-lym 360 umol/L
致癌性:
5,5-二苯基乙内酰脲 修改号码:5
模块 11. 毒理学信息
IARC = 2B (怀疑对人类致癌)。
NTP = b(合理预期致癌物)
生殖毒性: 无资料
RTECS 号码: MU1050000
模块 12. 生态学信息
生态毒性:
鱼类: 96h LC50:>16.0 mg/L (Oryzias latipes)
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 1 % (by BOD), 2 % (by HPLC)
潜在生物累积 (BCF): 1.7 (conc. 0.1 mg/L), <5.0 (conc. 0.01 mg/L)
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constant(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A